India’s pharmaceutical industry is a global powerhouse, contributing 20% of the world’s
generic medicines and ranking 3rd worldwide in terms of production volume. However,
as global demand grows and regulations tighten, Indian pharma firms face rising pressure to
maintain quality, efficiency, and compliance.
One strategic way forward? Deploying robotic solutions—particularly advanced quadruped
and humanoid robots like those from Unitree.
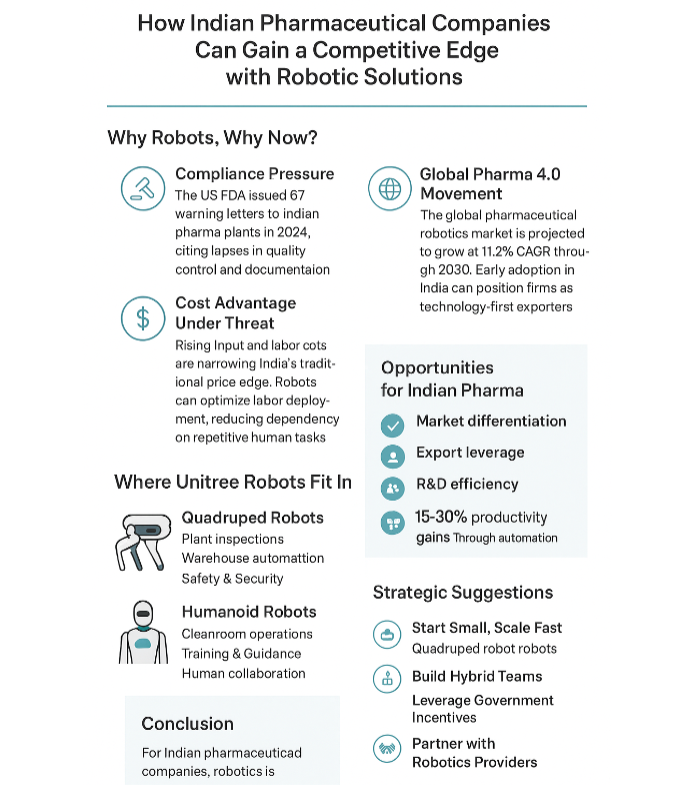
Why Robots, Why Now?
1. Compliance Pressure
The US FDA issued 67 warning letters to Indian pharma plants in 2024,
citing lapses in quality control and documentation.
Robots reduce human error and ensure precise, auditable processes.
2. Cost Advantage Under Threat
Rising input and labor costs are narrowing India’s traditional price edge.
Robots can optimize labor deployment, reducing dependency on repetitive
human tasks.
3. Global Pharma 4.0 Movement
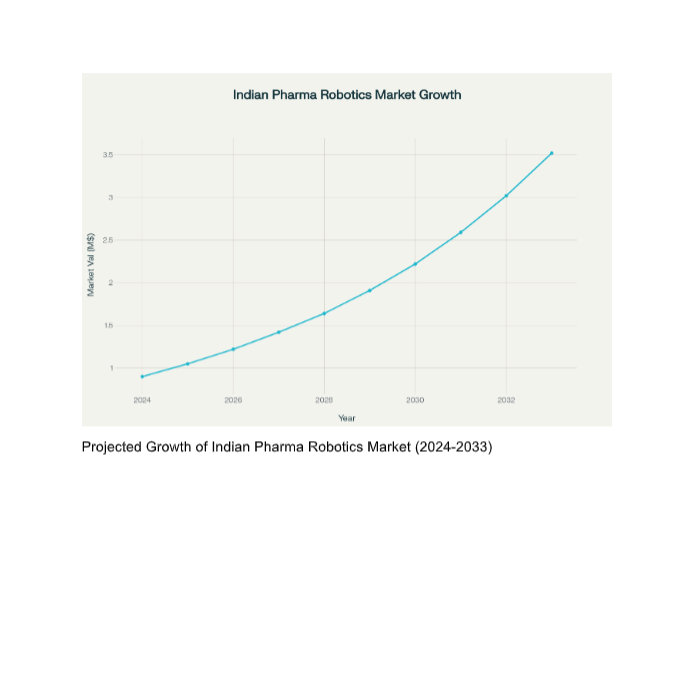
The global pharmaceutical robotics market is projected to grow at 11.2%
CAGR through 2030.
Early adoption in India can position firms as technology-first exporters.
Where Unitree Robots Fit In
1. Quadruped Robots
Plant Inspections: Navigate factory floors and hazardous areas to detect anomalies in
temperature, humidity, or chemical leaks.
Warehouse Automation: Carry materials between production zones, ensuring
compliance with sterile handling.
Safety & Security: Monitor restricted access zones with thermal and vision sensors.
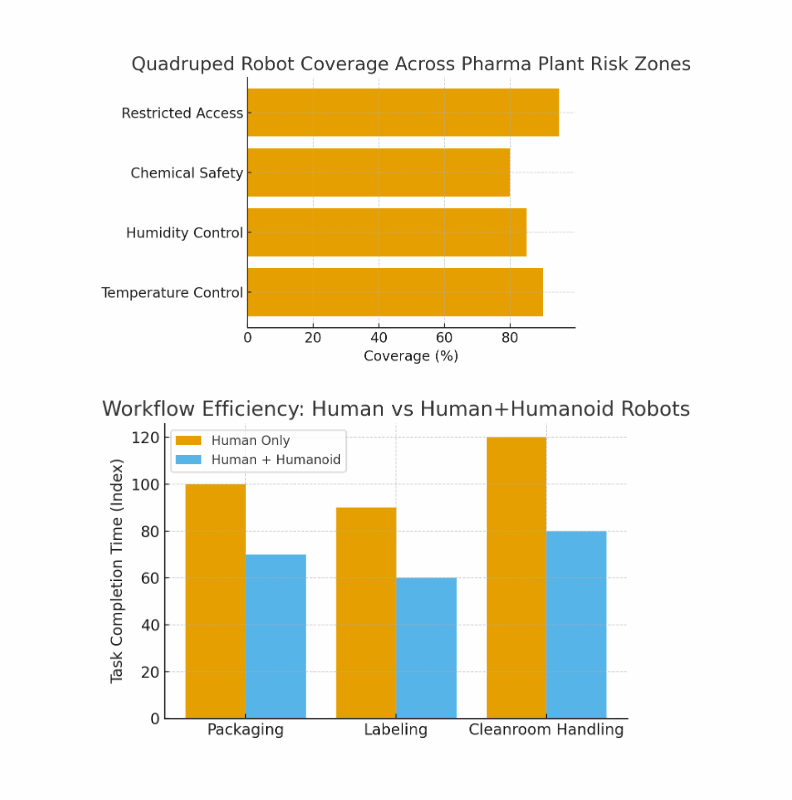
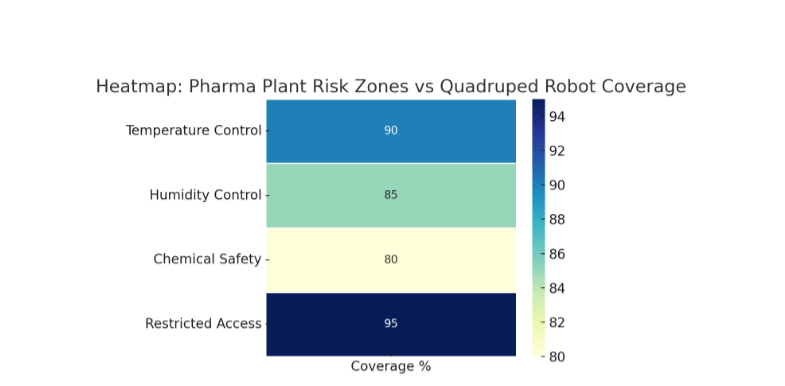
Heatmap chart of pharma plant risk zones vs. quadruped robot coverage.
2. Humanoid Robots
Cleanroom Operations: Execute delicate tasks like weighing, mixing, or transferring
APIs under sterile conditions.Training & Guidance: Act as interactive digital trainers for new staff in GMP
(Good Manufacturing Practices).
Human Collaboration: Assist in repetitive packaging and labeling, ensuring fewer
rejects.
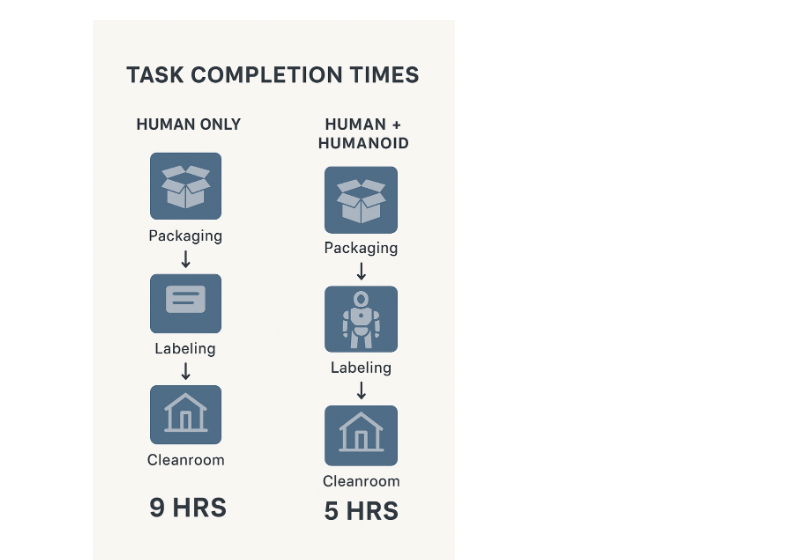
Workflow diagram comparing human-only vs. human+humanoid task completion times.
Opportunities for Indian Pharma
Market Differentiation:
Being early adopters boosts credibility with regulators and
clients.
Export Leverage: Pharma buyers in the US/EU increasingly demand digitally
monitored, automated facilities.
R&D Efficiency: Robots can handle repetitive assays, freeing scientists for higher-
value innovation.
Stat Spotlight: A McKinsey study suggests pharma companies could achieve 15–30%
productivity gains through automation.
Challenges to Consider
High CAPEX: Unitree’s humanoids cost upwards of $90,000–$150,000 per unit.
Integration Complexity: Robots must interface with MES (Manufacturing Execution
Systems) and ERP software.
Workforce Resistance: Fear of job displacement may slow adoption.
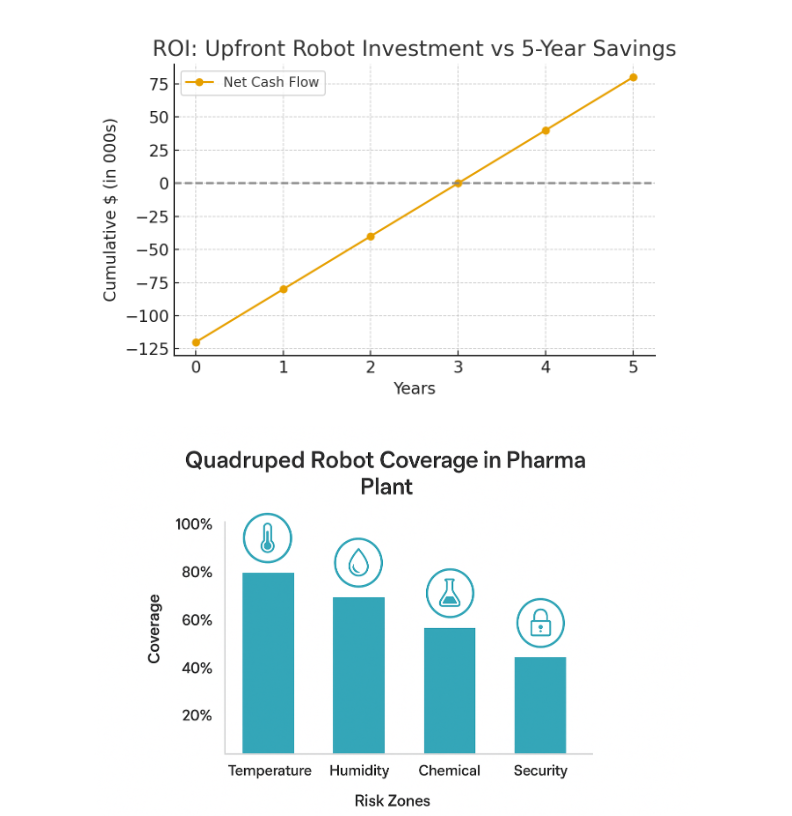
Visual Idea: Bar chart comparing upfront robot investment vs. 5-year cost savings in labor +
compliance penalties avoided.
Strategic Suggestions
1. Start Small, Scale Fast
Pilot quadrupeds in environmental monitoring and safety patrols—quick
ROI use cases.
2. Build Hybrid Teams
Pair humanoid robots with skilled operators in packaging and cleanroom
settings.
3. Leverage Government Incentives
Tap into PLI (Production Linked Incentive) schemes, which reward
automation-led productivity.
4. Partner with Robotics Providers
Collaborate with Unitree distributors for custom pharma-grade adaptations
(sterilizable casings, GMP certifications).
Conclusion
For Indian pharmaceutical companies, robotics isn’t just about replacing labor—it’s about
gaining a competitive edge in global markets. By deploying Unitree’s quadrupeds and
humanoids strategically, firms can:
Improve compliance and audit readiness
Cut costs and reduce operational risks
Signal innovation to global buyers
The companies that move first will likely set the industry standard for the next decade.
1. Quadruped robot coverage across pharma plant risk zones (bar chart).
2. Workflow efficiency comparison: Human-only vs Human+Humanoid robots.
3. ROI projection: Upfront investment vs. 5-year cumulative savings.